Medical Device Testing Strategies Implementation: A High-Level Overview
time2022/03/31
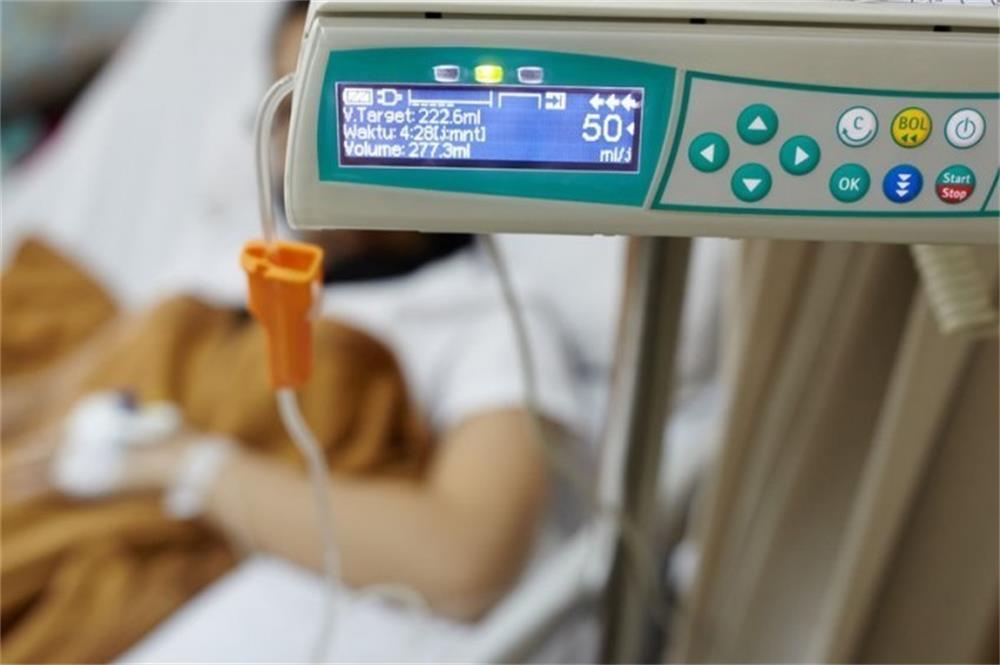
- Medical devices are crucial since they directly impact human lives. Medical device manufacturers must follow testing, verification, and validation best practices to ensure quality and reliability of medical equipment in order to provide safe and effective healthcare services to patients. Here is a high-level overview of implementing medical device testing strategies.
Medical Device Testing Strategy Implementation: A High-Level Overview
Medical devices are vital because they have a direct impact on human life. In order to provide safe and effective healthcare to patients, medical device manufacturers must follow testing and verification and validation practices to ensure the quality and reliability of medical devices. The following is a high-level overview of implementing a medical device testing strategy.
Medical devices are vital because they directly affect human life. Medical device manufacturers must follow testing, verification and validation best practices to ensure the quality and reliability of medical devices in order to provide safe and effective healthcare to patients. The following is a high-level overview of implementing a medical device testing strategy.
Multiple regulatory agencies and compliance govern medical devices. On the other hand, end users expect superior performance, efficacy and security from the devices they use. This forces medical device manufacturers to create and deploy medical device testing strategies throughout the entire development cycle – from concept to design to production.
A medical device testing strategy must include compliance processes and technical testing methods to improve the performance and effectiveness of medical devices. Manufacturers should develop a robust test plan from the very beginning of the design process, as exhaustive testing of finished products is ineffective and inefficient.
For example, to improve test coverage, manufacturers must test every function of a medical device from the design stage. This ensures cost and time efficiencies that are difficult to achieve when testing manufactured devices.
Develop an effective medical device testing strategy
The test team should consult the design team as a source of knowledge. Design input helps derive test structures that match hardware, software, or other technical requirements. To determine the risk mitigation testing needs of a device, a design class pattern, effect, and criticality analysis (FMECA) can be used.
An effective medical device testing strategy requires multiple sets of testing requirements.
These test requirements are based on the device's component specifications, manufacturing process and other critical functional specifications. Test requirements define and describe the setup conditions, actions, and expected response constraints for each experiment defined in the test step.
These sets of requirements are needed to make test implementation easier as testing occurs continuously at various stages throughout the manufacturing process, from component selection to final assembly of the medical device. Each stage has its own set of requirements and parameters to meet.
Featured related content
Learn how embedded IoT medical devices work
Applied Medical Device Testing Strategies
Production testing of components, sub-assemblies and finished products is included in the highest level of medical device testing strategies for technical testing. A test strategy considers the specific hardware and software requirements of each test phase, as well as the measurement methods and expected outputs.
An effective testing strategy is a production-level activity that considers the complete test suite at every step of the product development process. It analyzes and correlates faults in test models to improve the overall performance of the device. The strategy defines the desired output of each stage to ensure overall efficacy. The system is broken into small blocks during the validation process to maintain traceability to the original testing strategy, and then testing starts with specific criteria for each block in the system. The verification method for each block is tailored based on risk-based analysis to better match the testing strategy. The high-level test strategy provides a strong reference for the technical review and validation of equipment.
Microprocessor test
Medical devices must undergo rigorous electronic testing to meet the highest quality standards. At the heart of most Class II and Class III medical devices is a microprocessor. Therefore, microprocessor evaluation is the first step in medical device testing. Access to transistor interconnects within a microprocessor is necessary for effective testing. However, to improve testing efficiency, test teams should test microprocessor chips before inserting them into printed circuit boards (PCBs).
Integrated circuits are tested to see how their logic gates function and how they are connected. Depending on the requirements, select the appropriate technique from a variety of industry-standard test methods.
Once all components have been connected to the PCB, the mounting and interconnection process is tested. Throughout this phase, the test team uses a common assembly defect model to find faulty components, missing components, open interconnects, shorted interconnects, and other issues during PCB assembly.
Modern test equipment provides physical access to the PCB and allows direct measurement of components in a panel. It is important to ensure that these small components in the PCB do not affect the functionality of the overall system. Therefore, functional testing is essential for all functions affected by other component parameters. Functional tests allow the use of similar PCBs, but they are not sufficient to find common manufacturing defects. Therefore, additional troubleshooting is required to determine specific repairs.
Overview of FDA Medical Device Regulations
automated test
A test automation system is an electronic system that includes computers, instruments, and software for carrying and controlling the test process. Certain commercial test automation systems are available in the market according to industry standards. However, test teams can use customized test systems according to their needs.
Testing teams may need to adjust strategies based on available testing alternatives at each step of medical device development. It may constrain the test team by restricting implementation techniques.
Test teams can face challenges in a test automation environment when testing complex medical devices with stringent voltage and current requirements. This is due to the limited ability to develop test cases and measure test accuracy.
Ideally, automated test implementation is a matter of doing both hardware and software design. The test set for a stage in the manufacturing process is determined by the specification assigned to that particular stage in the test strategy. We must decompose these test specifications into software and hardware requirements specifications in terms of test system software and interface hardware, respectively.
System specifications usually originate from the medical device design stage, but certain requirements, such as security and data integrity, must originate from the production test environment. All of these standards lead to the mechanical design, coding and electrical design of the test system. In an established facility that has designed, manufactured and tested multiple medical devices, reuse common hardware and software blocks to simplify the process.
verification process
Once everything is in place, the medical device test system must be validated, both software and hardware. The software and hardware verification process for medical devices is specific. The purpose of validation is to test whether the device meets the needs of a specific user. Therefore, structures and methods are crucial for applying verification methods.
Validation takes place on the first production unit. In other words, verification equipment is integrated into the production environment. The process involves end users and is tested under simulated or actual use. Validation testing is necessary to ensure that medical devices work as intended and meet the needs of their users.
The initial approach to verification starts with unit testing at a set of stages in the process, and then verifies the complete system. Given the complexity of the system, most automated systems find verification challenging. In this case, a divide-and-conquer verification approach would be better.
Integration testing of the entire system is a critical technique, but it should be the result of a coordinated verification protocol, not the entire program.